This clinical trial with Edvince lead molecule is an interventional, randomised, double-blind, placebo-controlled, multiple-ascending-dose, first-in-human trial of the MEK1/2 inhibitor EDV2209 in patients with Subarachnoid Hemorrhage – SAH (EudraCT no. 2021-003629-31). The primary objective is to investigate the safety and tolerability of the study drug in patients diagnosed with subarachnoid hemorrhage and in need of an intraventricular drain. The study drug is administered locally via the drain which is installed to control the pressure caused by the massive bleeding.
Since the trial is conducted in SAH patients, clinical outcome scales are included as secondary endpoints at 2 and 12 weeks after the hemorrhage (National Institute of Health Stroke Scale (NIHSS); modified Rankin Scale (mRS), and Extended Glasgow Outcome Scale (GOS-E)). Multiple doses of study drug are tested in approximately 30 patients, with most patients at the highest dose. This is to allow for analysis of the clinical endpoints in the high-dose group where a clinical difference between patients on active study drug versus placebo is expected. The study takes place at the Neurointensive Care Unit at Rigshospitalet, Copenhagen (DK) and the estimated recruitment period is 12-18 months. Additional sites in Denmark and Norway may be opened subsequently to secure patient recruitment within the timeline.
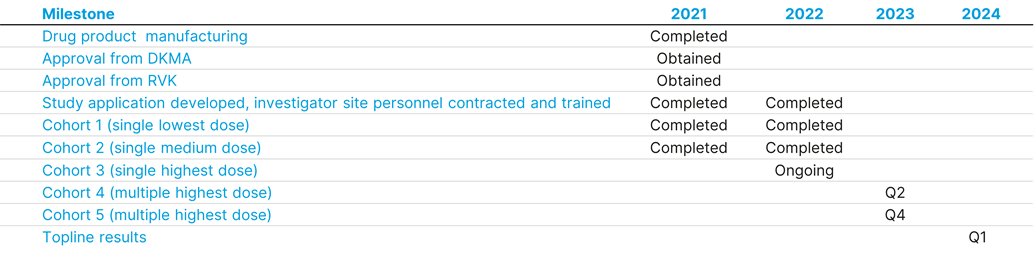
The recruitment of patients is expected to be completed in 2023 with top line results in Q1 2024.